States of matter quiz
15 QuestionsQuiz Description
In this exciting quiz, we are going to be looking at states of matter. Here, you will be trained on answering questions based on your understanding of the various states such as the solid, liquid, and gaseous states, as well as the conversion from one state to the next. This quiz is worth giving it a shot.
Matter as we all know is anything that has mass and occupies space. Furthermore, matter can exist in different forms known as states which are: solid, liquid, and gaseous states. Also, matter can exist as plasma, which is a state of matter obtained after heating it up in such a way that it releases electrons forming gaseous ions. It is often known as the fourth state of matter.
Matter can also undergo some changes of state like from solid to liquid, liquid to gas, etc. An example could be the change of state of water (ice) from solid to liquid (melting), then from liquid to gas (evaporation). It could also move from gas to liquid (condensation), and from liquid to solid (solidification).
Some matter could even change from solid to gas directly (sublimation) or from gas to solid (deposition).
This quiz is made available for chemistry students willing to excel in chemistry. Hence, if you are that student, take the quiz and train yourself. Good luck as you practice.
The table below shows the melting points and boiling points of substances A to D. Which substance is a solid at 100oC? [Substance] Melting point oC(mpt), Boiling Point oC(bpt)
The table below shows the melting points and boiling points of substances A to D. Which substance is a liquid at 25oC? [Substance] Melting point oC(mpt), Boiling Point oC(bpt)
Which of the following statements best describes the arrangement of particles in a solid?
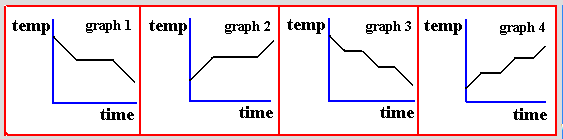
Which of the following graphs represents how the temperature of liquid varies as it becomes a solid?
When water vapour in a cloud is gradually cooled, rainwater is formed. This change of state is called?
When the air in a room becomes cooler a hanging balloon blown up with air becomes smaller because the molecules of air?
Which of the following best describes what happens to the particles of ice when it melts?


