Reversible Reactions and Ammonia Quiz
15 QuestionsQuiz Description
The quiz, reversible reactions and ammonia is made up of 15 questions which have been set based on the national curriculum for the gcse level and have been set to meet up the gcse examination standard with varying difficulty levels. Out of the four options in each of the questions, there is one and only one of the options that is correct so take your time and think before choosing an answer.
In this particular quiz, we have three main terms that we need to define and they are; reaction, reversible reaction and ammonia.
A reaction is a process whereby at least one substance (known as the reactants) is/are changed to another unique substance (known as the product).
A reversible reaction is therefore a reaction whereby the reactants converted to the products can undergo the transformation of the products into the reactants.
Ammonia, which is generally known as NH3 is a compound which is used in the creation of urea, rayon and other things
Other quizzes have been made available for here on this platform, click HERE to access them. With this, you can now go ahead and start answering the questions below.
Good luck
Which is TRUE about reversible reactions that have formed a chemical equilibrium?
Which ratio numbers a, b and c are needed to balance the equation showing the neutralisation of ammonia to form ammonium nitrate?
a NH3(aq) + b HNO3(aq) ==> c NH4NO3(aq)
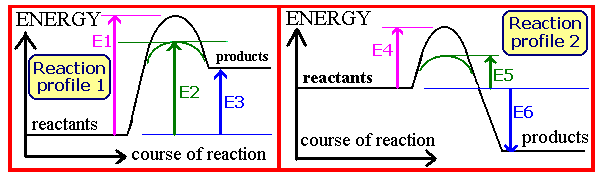
On the reaction profile diagrams above, which energy change corresponds to the activationed energy for the iron catalysed exothermic formation of ammonia?
Which is TRUE about reversible reactions that have formed a chemical equilibrium?
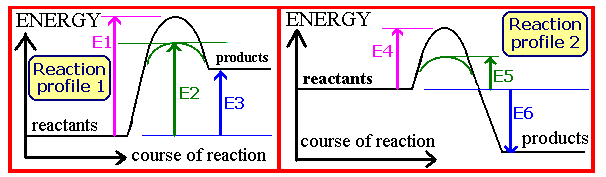
On the reaction profile diagrams above, which energy change corresponds to the overall energy change for the uncatalysed endothermic thermal decomposition ammonium chloride?
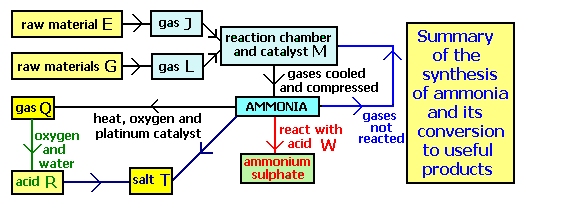
Which is needed to bring about the conversion of ammonia into nitrogen monoxide?
In the synthesis of ammonia what happens to the unreacted nitrogen and hydrogen?
Which ratio numbers a, b and c are needed to balance the equation showing the neutralisation of ammonia to form ammonium sulphate?
a NH3(aq) + b H2SO4(aq) ==> c(NH4)2SO4(aq)
Which of these is a problem caused by the over-use of artificial fertilisers like ammonium nitrate on fields of crops?