Rates of Chemical Reaction Quiz
18 QuestionsQuiz Description
Rates of chemical reaction quiz which has been made available to you for free is made up of questions which meet the examination requirements for students in the gcse level but here we're going to briefly talk about rates of chemical reactions.
Chemical reactions are the changes which are made to the arrangement of the atoms and molecules found in materials. Chemical reactions need different lengths of time to come to an end and this depends on the characteristics of the reaction reactants, the characteristics of its products and the conditions under which the reaction has to take place.
When you’re done with this quiz, rates of chemical reaction quizzes, you will be able to tackle any question that comes from this particular aspect of chemistry with absolute ease. So you can now go ahead and test your knowledge with the questions below and it is also important to take note that several quizzes have been made available for you here at gcequiz.com.
Good luck
Iron reacts with hydrochloric acid to form iron(II) chloride and hydrogen gas. 2g of iron is added to 100cm3 of dilute hydrochloric acid. Which conditions produces the fastest reaction?
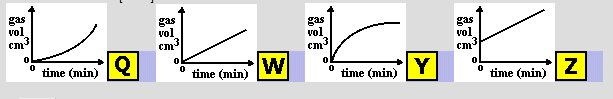
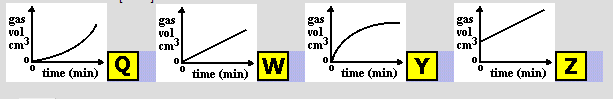
A gas was produced in a chemical reaction. The total volume of gas formed was measured at regular time intervals. Which graph would you expect the results to be like?
Limestone or marble chips are made of the chemical calcium carbonate (1). They both dissolve in hydrochloric acid (2), to produce a solution of calcium chloride (3), water (4) and carbon dioxide (5). Which of 1 to 5 is the 'salt' in the reaction?
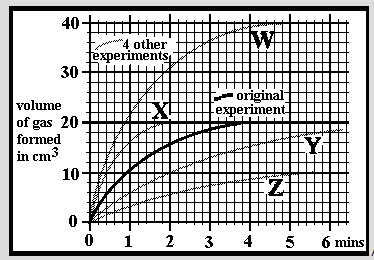
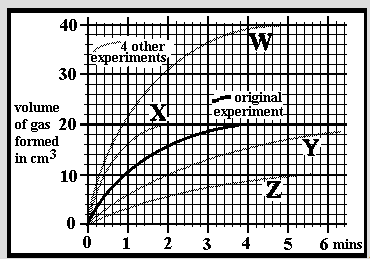
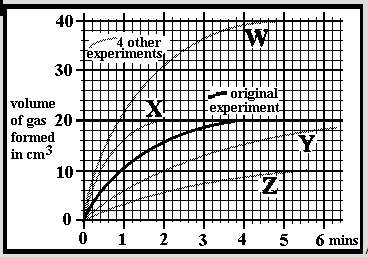
An experiment was carried out at a temperature of 25oC by dissolving 0.2g of small limestone granules into 30cm3 of hydrochloric acid. The acid concentration was 1.0 mol/dm3 and in excess so all the limestone dissolved. The reaction was followed by measuring the volume of carbon dioxide given off over a period of 6 minutes. A 2nd experiment was done in an identical manner but using 0.2g of limestone lumps. Which of the graphs W, X, Y or Z might you expect for the results?
A large amount of calcium carbonate (excess) is added to a small amount of dilute hydrochloric acid. Which will double the volume of carbon dioxide finally formed?

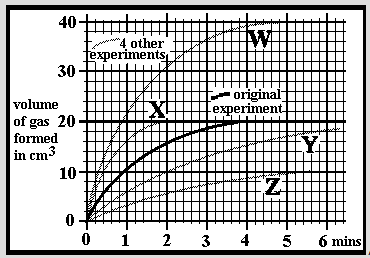
An experiment was carried out to dissolve small limestone granules into hydrochloric acid. Carbon dioxide gas is formed and was collected in a gas syringe. The initial results are marked as the 'original experiment'. The experiment was repeated after varying the reaction conditions (ie mass of limestone, size of limestone pieces, temperature or acid concentration). The speed of the reaction can be given in cm3/min, ie the speed at which carbon dioxide is formed. Which experiment is going at an approximate average speed of 6 cm3/min in the first minute of the reaction?
For reactions to occur, the particles of the reactants must collide. The fewer the collisions that take place, the slower the rate of a reaction. Which DECREASES the frequency of collision between reactant particles?
A small quantity of calcium carbonate is added to a large excess acid. Carbon dioxide gas is formed. Which of the following will halve the amount of carbon dioxide formed?
Limestone or marble chips are made of the chemical calcium carbonate (1). They both dissolve in hydrochloric acid (2), to produce a solution of calcium chloride (3), water (4) and carbon dioxide (5). In what form would you add the marble to slow down the reaction?
For reactions to occur, the particles of the reactants must collide. The fewer the collisions that take place, the slower the rate of reaction. Which INCREASES the frequency of collision between reactant particles?
An experiment was carried out at a temperature of 25oC by dissolving 0.2g of small limestone granules into 30cm3 of hydrochloric acid. The acid concentration was 1.0 mol/dm3 and in excess so all the limestone dissolved. The reaction was followed by measuring the volume of carbon dioxide given off over a period of 6 minutes. A 2nd experiment was done in an identical manner but using 0.4g of the same limestone granules. Which of the graphs W, X, Y or Z might you expect for the results?
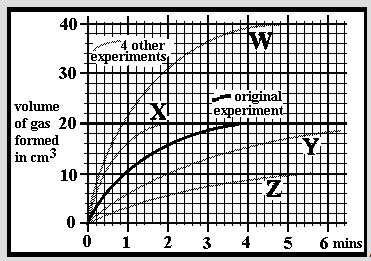
An experiment was carried out to dissolve small limestone granules into hydrochloric acid. Carbon dioxide gas is formed and was collected in a gas syringe. The initial results are marked as the 'original experiment'. The experiment was repeated after varying the reaction conditions (ie mass of limestone, size of limestone pieces, temperature or acid concentration). The speed of the reaction can be given in cm3/min, ie the speed at which carbon dioxide is formed. Which experiment is going at an approximate average speed of 3 cm3/min in the first minute of the reaction?
Calcium carbonate in the form of limestone dissolves in hydrochloric acid and giving off carbon dioxide gas. Which conditions would produce the fastest reaction?
An experiment was carried out at a temperature of 25oC by dissolving 0.2g of small limestone granules into 30cm3 of hydrochloric acid. The acid concentration was 1.0 mol/dm3 and in excess so all the limestone dissolved. The reaction was followed by measuring the volume of carbon dioxide given off over a period of 6 minutes. A 2nd experiment was done in an identical manner but using 0.1g of the same limestone granules. Which of the graphs W, X, Y or Z might you expect for the results?
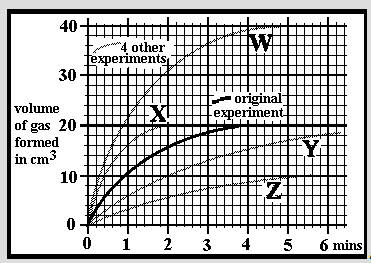
An experiment was carried out at a temperature of 25oC by dissolving 0.2g of small limestone granules into 30cm3 of hydrochloric acid. The acid concentration was 1.0 mol/dm3 and in excess so all the limestone dissolved. The reaction was followed by measuring the volume of carbon dioxide given off over a period of 6 minutes. A 2nd experiment was done in an identical manner but using acid of 2.0mol/dm3 concentration. Which of the graphs W, X, Y or Z might you expect for the results?
On mixing two reacting solutions a solid precipitate was formed. The mass of the solid precipitate was measured at regular time intervals. Which graph would you expect the results to be like?