Methods of Purification and analysis quiz
15 QuestionsQuiz Description
In this chemistry quiz on methods of purification and analysis, we are going to be looking at some important practical scenarios in chemistry such as the identification of the presence of a substance in a mixture, an understanding of experimental parameters, the separation of mixtures, and so on. This quiz is loaded with the right information for you to study with. You should give it a go.
All pure substances have their respective properties which are unique to them, such as boiling and melting points, solubility, etc. These properties are used to determine a particular method of separation of a mixture between two pure substances. For example, fractional distillation is used to separate a mixture of liquids, making use of their respective boiling points. Before going any further, let’s know what a mixture is:
A mixture is the combination of two or more pure substances. It could either be homogeneous (meaning the pure substances in the mixture are all in the same physical state) or heterogeneous (meaning the pure substances in the mixture are in different states).
Having a glance at this quiz already makes it reveal its potential. If you are willing to study this exciting topic in chemistry, go through the quiz and test yourself. Wish you the best as you practice with us.
Which property of a liquid ester can be used to check its purity before use as a food flavoring?
The apparatus shown is used to distil a dilute solution of ethanol in water. [B.P.: ethanol, 78 °C; water 100 °C]
![<p>The apparatus shown is used to distil a dilute solution of ethanol in water. [B.P.: ethanol, 78 °C; water 100 °C]</p>](https://v1.api.gcequiz.com/media/image/5ef6f3251cc3cc54778de5ac.png)
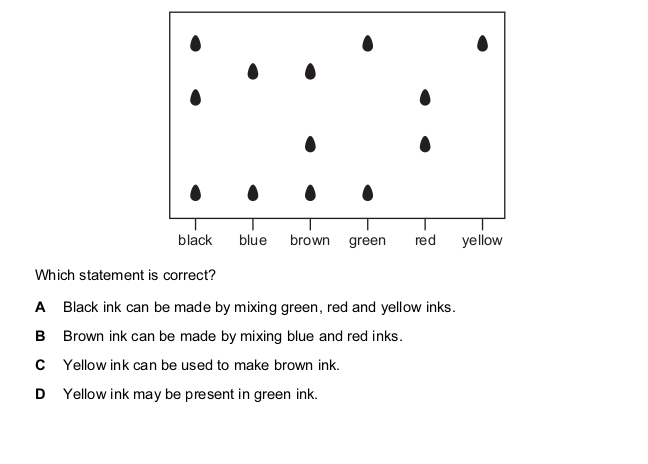
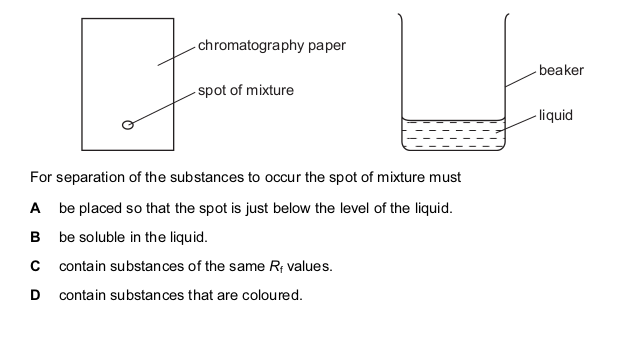
A mixture of two substances is spotted onto a piece of chromatography paper.
The paper is inserted into a beaker containing a liquid.
It is suspected that a lollipop contains traces of a poisonous green dye (boiling point 73 °C) as well as two harmless orange and red dyes (boiling points 69 °C and 73 °C respectively).
What is the best method by which the green dye may be detected?

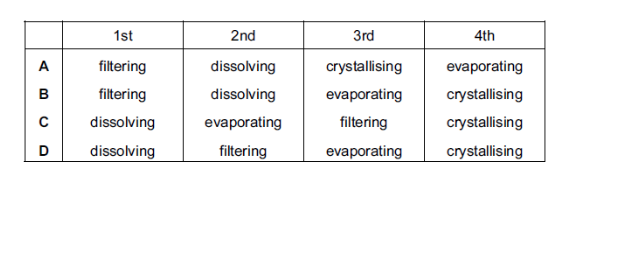
Copper(II) sulfate crystals are separated from sand using the four processes listed below.
In which order are these processes used?
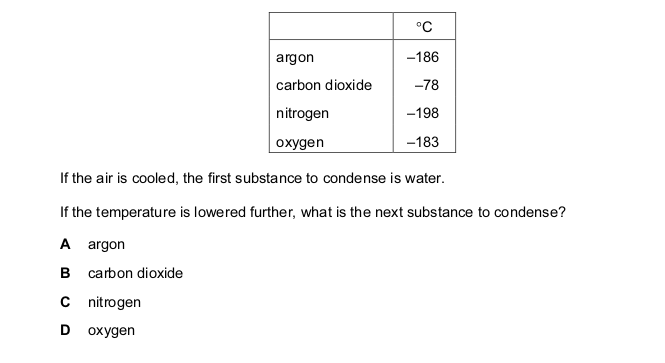
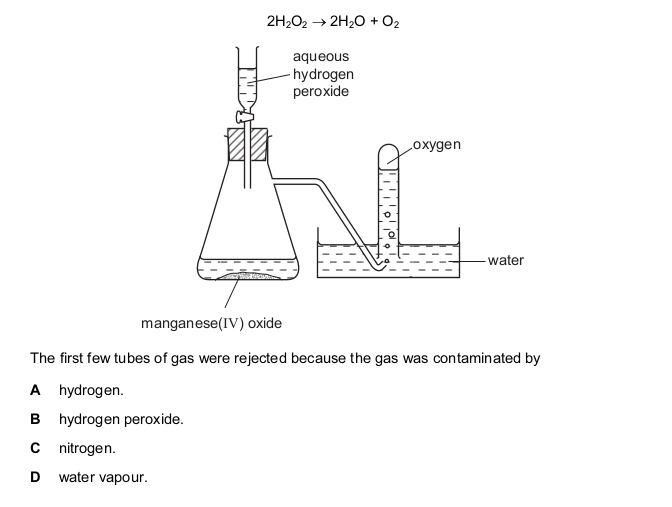
Oxygen was prepared from hydrogen peroxide, with manganese(IV) oxide as catalyst. The
oxygen was collected as shown in the diagram.
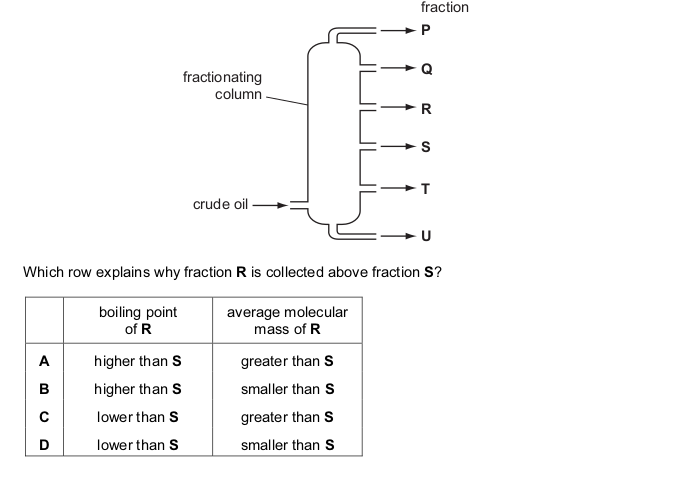
The two statements are about the fractional distillation of crude oil. The statements may or may not be correct. They may or may not be linked.
statement 1: Fractional distillation is used to separate crude oil into useful fractions.
statement 2: The fractions with lower boiling points are found at the top of the fractionating column.
What is correct about these two statements?
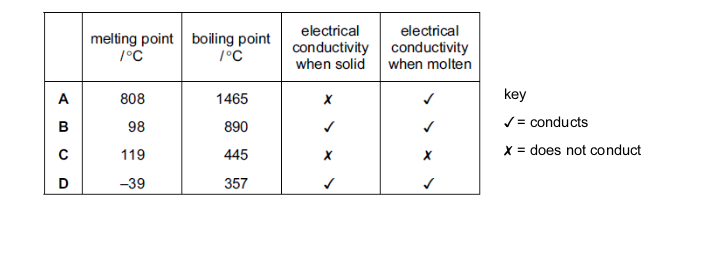
The table gives the properties of four substances.
Which substance is a solid metal at room temperature?
Which method could be used to obtain charcoal from a mixture of powdered charcoal with sodium chloride?