Identification of Ions and Gases Quiz
15 QuestionsQuiz Description
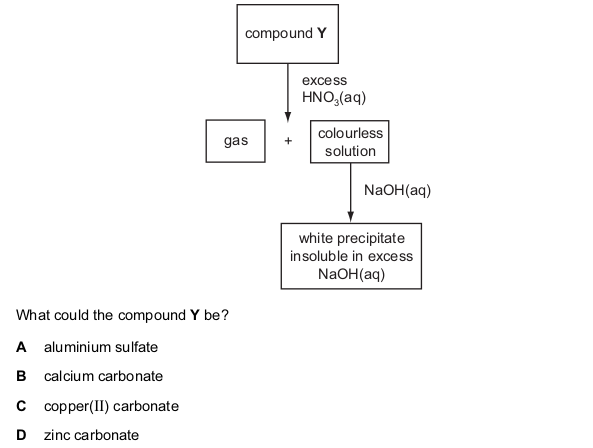
One very important aspect of gcse chemistry is the identification of ions (which is an atom or molecule having a net electrical charge, this charge could be negative as in the case of an electron, positive as in the case of protons or just neutral). gcequiz.com has brought together questions in the identification of ions and gasses as seen in the gcse. Questions that are up to examination standards.
There are so many ways to test the presence of an ion in a gas or in a solution. Some of these methods include flame tests, it’s reactivity with some metal wires such as nichrome or platinum.
Apart from chemistry quizzes, other quizzes have been made available for you on this platform to ease your studies and preparations for the end of year exams.
Good luck
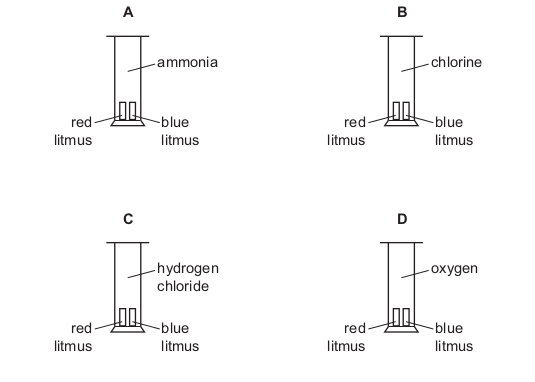
Four gas jars each contain one of the gases ammonia, chlorine, hydrogen chloride and oxygen. A
strip of damp blue litmus paper and a strip of damp red litmus paper are placed in each jar.
In which gas jar will both the damp blue litmus paper and the damp red litmus paper change
colour?
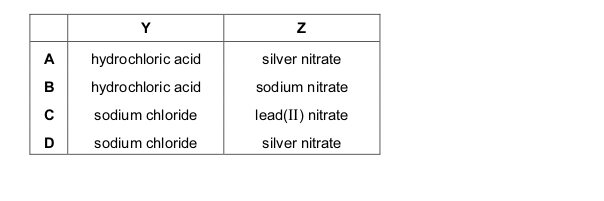
A student mixed together aqueous solutions of Y and Z. A white precipitate formed.
Which could not be Y and Z?
A liquid reacts with each of sodium carbonate, potassium hydroxide and ethanol. What is the liquid?
Which compound when in aqueous solution will produce a red / brown precipitate on the addition of an aqueous solution of Fe 3+ ions?
In which pair do neither of the gases change the colour of damp blue litmus paper?
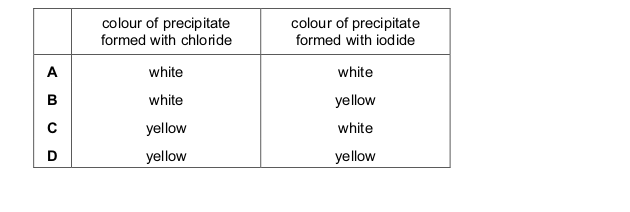
Aqueous silver nitrate is added to separate solutions of potassium chloride and sodium iodide.
What are the colours of the precipitates formed?
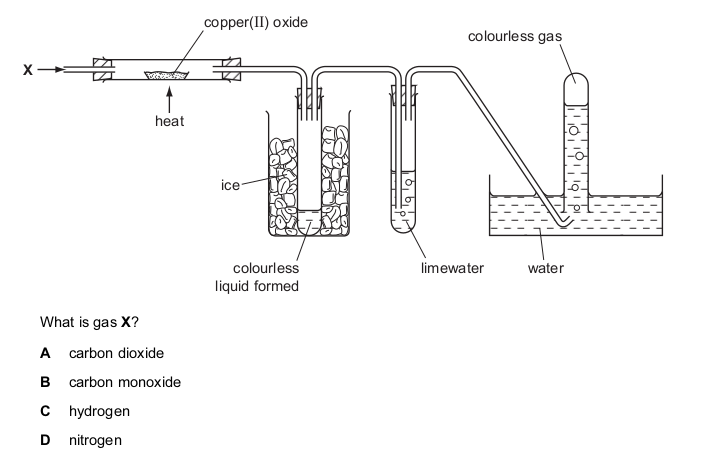
When pure gas X was passed through the apparatus shown, the copper( II ) oxide turned pink and the lime water stayed colourless.
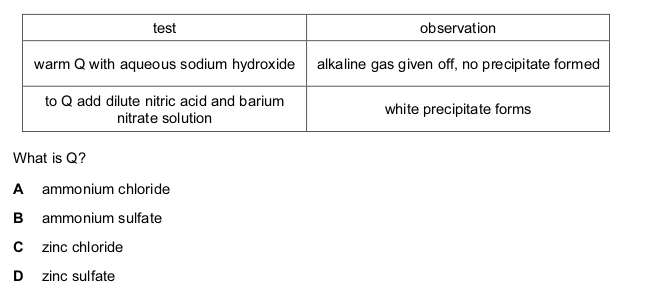
The addition of dilute acid to a solution containing the anion Q and the subsequent use of limewater can be used to identify the anion Q.
What is Q?
Which ion reacts with aqueous ammonia to give a precipitate that dissolves in an excess of ammonia?
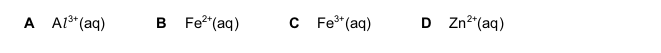
A mixture of two gases has no effect on either damp blue litmus paper or damp red litmus paper.
Which gases are present in the mixture?