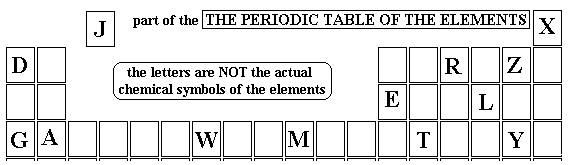
Group I Alkali Metals Beginner Quiz
15 QuestionsQuiz Description
In gcse chemistry, it is of most importance that students be able to know all the group I elements with some physical and chemical properties that are peculiar about elements found in this group (i.e from Lithium (Li) to Francium (Fr)).
Group I elements, also referred to as the Group 1 elements by the IUPAC System and also referred to as the Alkali Metals are the chemical elements found in the first column of the periodic table, with the elements, Lithium (Li), Sodium (Na), Potassium (K), Caesium (Cs), Rubidium (Rb) and Francium (Fr).
These elements are characterized by 1 electron found on the valence shell of it’s atom, they react vigorously with water to produce a solution known as an alkaline solution. Our quizzes here at gcequiz.com are free of charge. With all this said, you can now go ahead and test your knowledge.
Good luck
Given the equation ...
2K(?) + 2H2O(?) ==> 2KOH(X) + H2(?)
Which state symbol should be where (X) is?
Which is TRUE about the reaction of potassium and water containing universal indicator?
Sodium carbonate solution is a weak alkali, the pH of its aqueous solution is likely to be?
Which is TRUE about the reaction of lithium and water containing universal indicator?
When an alkali metal reacts with the non-metal chlorine, which statement is TRUE about the compound formed?
Given the equation ...
2Li(X) + 2H2O(?) ==> 2LiOH(?) + H2(?)
Which state symbol should be where (X) is?
Which is TRUE about the compound formed on combining an Alkali Metal with the element oxygen to form the metal oxide?
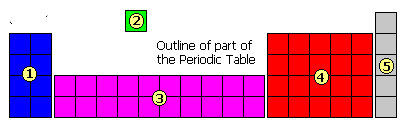
The diagram shows an outline of part of the Periodic Table in five sections. In which section will you find hydrogen?
Given the equation ...
2Na(?) + 2H2O(X) ==> 2NaOH(?) + H2(?)
Which state symbol should be where (X) is?
When an alkali metal reacts with the non-metal chlorine, which statement is TRUE about the compound formed?