Atomic structure and isotopes quiz
15 QuestionsQuiz Description
In this quiz, we shall cover the concept of atomic structures and isotopy. One important aspect we are going to be looking at is valency. You’ll get to be good at understanding the structure of the atom, the arrangement of elements on the periodic table, isotopy, etc. This is a quiz you should try out by yourself.
Isotopy is the existence of atoms of the same elements having the same atomic numbers, but different mass numbers due to the difference in their number of neutrons. This, therefore, means that isotopes are two or more atoms of the same element having the same atomic number but different mass numbers. It is known to be true that every element has one or more isotopes.
Talking about an atom, it is the smallest unit to which matter can be split without the release of electrically charged particles (protons and electrons).
Atomic structure and isotopy are very important to the study of chemistry. Do you want to train yourself to become a chemistry genius? Make out time to go through this quiz as well as the others. Good luck!
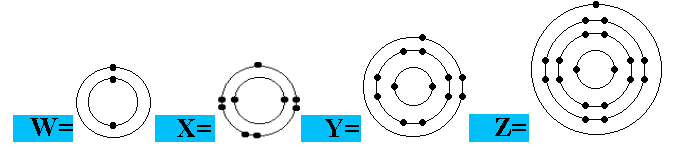
Which electron arrangements correspond to two elements in the same Group of the Periodic Table?
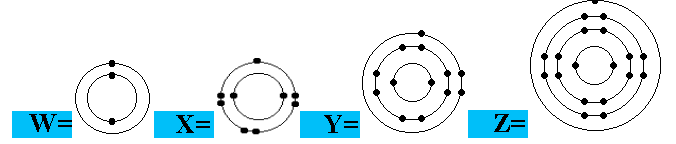
Which electron arrangements correspond to two elements on the same period of the Periodic Table?
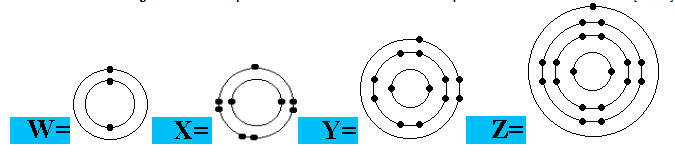
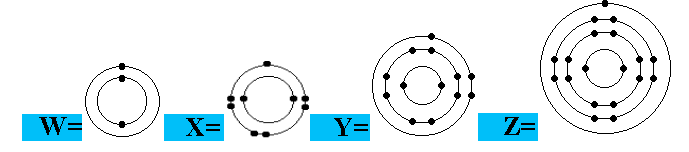
Which electron arrangement corresponds to a metal in Group 3 of the Periodic Table?