Acids, Bases, Salts and pH Quiz
15 QuestionsQuiz Description
Acids, bases and salts are very important in manufacturing and also for everyday life. They are used for the manufacture of things like soap, detergents, fertilisers, medicines and even car batteries. In this quiz, you have been provided with a question with four options and just one and only one is correct. It is important to note that these questions have been set based on the national curriculum for the gcse level.
An acid is a hydrogen-containing substance which is capable of donating a proton (which is a hydrogen ion) to another substance. On the other hand, a base is a substance which is capable of accepting the hydrogen ion from an acid. A base is characterized by its slippery texture and bitter taste. Salts are ionic compounds which result from a reaction between a base and an acid (known as a neutralization reaction). With this, salts are made up of cation and anions. pH is a measure of the acidity or basicity of a substance. The pH scale runs from 0 to 7 for acids and 7 to 14 for bases with 7 for neutral substances.
Other quizzes have been made available for here on this platform, click HERE to access them. With this, you can now go ahead and start answering the questions below.
Good luck
When sulfuric acid is added to zinc, the gas bubbles formed can be identified by?
In the reaction illustrated, in order to add the correct amount of acid you would add a few drops of Universal Indicator to the conical flask. If too little acid was added the indicator colour could be?
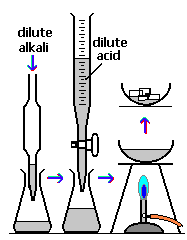
A carbonate mineral rock was tested with dilute sulfuric acid and bubbles formed. The gas formed was?
An iron tray was cleaned with dilute hydrochloric acid and bubbles formed. The gas formed was?
Which of A to D completes the equation ..?..
sulfuric acid + ..?.. ==> calcium sulfate + water
Insoluble bases and metals will not dissolve to form an alkaline solution, but they will dissolve in acids to form salts. This method is particularly handy for making transition metal salts. What pair of chemicals will make cobalt chloride and hydrogen?
A small amount of a dense white powder was mixed with water in a test tube. Non of the white powder sank to the bottom of the test tube. A few drops of universal indicator were added and the solution turned blue. This meant the white powder was ...?