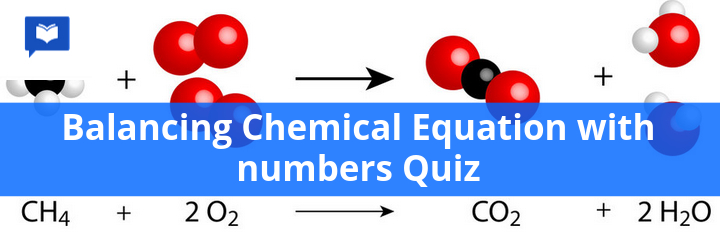
Balancing chemical equation with numbers quiz
Quiz Description
Revising Balancing chemical Equation with numbers using Quizzes
(Balancing chemical equation with numbers quiz for those students going in for the GCE A levels, GCSE and necta)
These quizzes are going to focus on what a chemical equation is some chemical equations that need to be balanced by using numbers. Here we're going to look at what a chemical equation is and how it is balanced using numbers, to be more specific, using natural numbers.
Chemical equations are emblematic portrayals of compound responses in which the reactants and the items are communicated regarding their individual synthetic formulae. They likewise utilize images to address factors, for example, the course of the response and the actual conditions of the responding substances. Chemical equations were first defined by the French scientific expert Jean Beguin in the year 1615.
We have as example the reaction between Hydrogen gas and oxygen gas to give water
H2 + O2 → H2O
Now to balance this chemical equation, we need to add numbers in front of the elements or compounds so that the number of elements on the right hand side of the equation is equal to the number of elements on the left hand side and by so doing, the chemical equation is balanced.
On the left hand side of the equation, we have 2 molecules of hydrogen and 2 molecules of oxygen.
On the right hand side of the equation, we have 2 Hydrogen and 1 oxygen. As you'll be going through our quizzes, you'll see quizzes that have to do with something related to this.
So we need to balance oxygen on the right hand side to have 2 oxygen on the left had side and two on the right hand side. By so doing, we have
2H2 + O2 → 2H2O
and this is balance
There are hundreds of thousands of quizzes here at gcequiz.com to help you practice and prepare for your exam. For more quizzes in other aspects of chemistry, click here.